- Article
Fe/Mn-Modified Biochar Facilitates Functional Microbial Enrichment for Efficient Glucose–Xylose Co-Fermentation and Biohydrogen Production
- Jianing Fan,
- Jiwen Wu and
- Ji Zhao
- + 4 authors
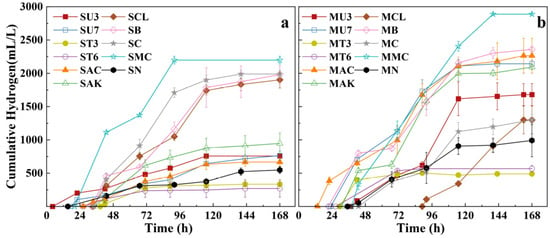
Biohydrogen production can be derived from low-value lignocellulosic biomass; however, in many biohydrogen producing systems, xylose is utilized less efficiently than glucose, which limits overall substrate conversion. To address this issue, Fe/Mn-modified biochar was employed to enhance dark fermentation of glucose–xylose mixed sugars, and its performance was compared with other inoculum treatments. The biochar addition achieved a hydrogen yield of 2.57 ± 0.10 mol-H2/mol-sugar, representing 14.6% enhancement over untreated controls, while enabling complete substrate utilization across varying xylose proportions. Biochar supplementation also reduced the lag phase by 24.4% and increased hydrogen productivity by 47.3% in mixed-sugar cultivation. Integrated analyses of the experimental data revealed the dual role of Fe/Mn-modified biochar in constructing conductive extracellular polymeric substance networks and directing metabolic flux toward high-yield butyrate pathways. This work establishes Fe/Mn-biochar as a multifunctional microbial engineering tool that alleviates carbon catabolite repression and promotes the enrichment of hydrogen-producing bacteria (HPB), thereby providing a practical and effective strategy for enhanced biohydrogen production from lignocellulosic biomass.
18 December 2025